The Most Accurate Test in the Market, Based on 2 Independent Validation Studies Across 53 Antigen Test Kits*
- To ensure accessibility, the United States FDA EUA authorized test will be one of the most affordable tests in the market at HKD60
- Flowflex™ will be available on projectscreen.co and in retail channels in CitySuper, Watsons, Mannings, HKTVMall and more
- Flowflex™ antigen test detects COVID-19, including the Omicron variant, in just 15 minutes with 98.8% accuracy
- Flowflex™ has been instrumental in the fight against COVID-19, with billions of tests used and available globally in 100+ countries, including the United States, United Kingdom, European Union, Singapore, Australia and now in Hong Kong.
HONG KONG, Feb. 25, 2022 /PRNewswire/ — Prenetics Group Limited (“Prenetics” or the “Company”), a global leader in genomic and diagnostic testing, announced today that it has partnered with ACON Bio to launch the Flowflex™ SARS-CoV-2 Antigen Rapid Test Kit (“Flowflex™“) in Hong Kong. The affordable and easy-to-use COVID-19 self-testing solution is the most accurate antigen test kit in the market*. FlowFlex™ is well-equipped with the latest patented technology and provides results in just 15 minutes with 98.8% accuracy.
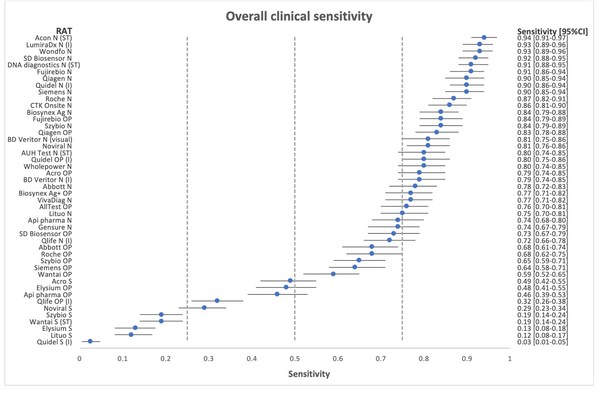
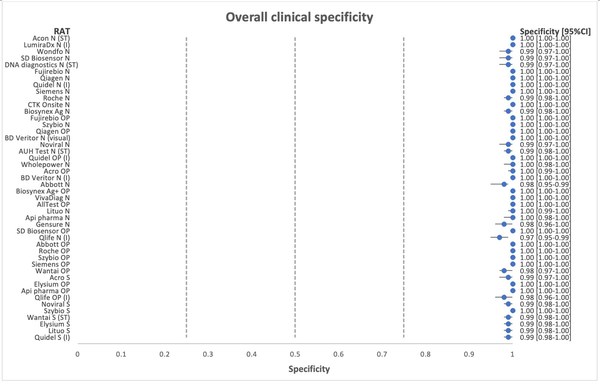
*Flowflex™ is the most accurate antigen test in the market in both clinical sensitivity and specificity, and also in the detection of Omicron, based on two independent validation studies:
Independent Nationwide Clinical Sensitivity and Specificity Study with 46 rapid antigen tests – Led by Copenhagen University Hospital, Denmark. In summary, FlowFlex™ achieved 94% sensitivity and 100% specificity in the study, outperforming all test kits, including from Roche and Abbott.
*Journal of Clinical Virology. A nationwife analytical and clinical evaluation of 46 rapid antigen tests for SARS-CoV-2 compared to RT-qPCR
https://www.hvidovrehospital.dk/presse-og-nyt/pressemeddelelser-og-nyheder/nyheder-fra-hvidovre-hospital/PublishingImages/Sider/Antigentests-svinger-voldsomt-i-kvalitet/A%20nationwide%20analytical%20and%20clinical%20evaluation%20of%2046%20rapid%20antigen%
Independent Clinical Omicron and Delta Sensitivity Study with 7 antigen tests – Led by the University of Geneva, Switzerland, and was supported by the Swiss National Science Foundation and the Foundation for Innovative New Diagnostics, an World Health Organization Collaborating Centre. In summary, FlowFlex™ achieved 91.2% and 88.9% sensitivity, respectively for the detection of Delta and Omicron variants, outperforming all antigen test kits.
|
Antigen Test |
Flowflex™ Antigen Rapid Test |
Panbio COVID-19 Ag Rapid test device |
Standard Q COVID-19 Ag |
Sure Status |
2019-nCoV Antigen test |
COVID-19 Antigen Rapid Test |
Onsite COVID-19 Ag Rapid Test |
|
Omicron Sensitivity |
88.9% |
36.1% |
22.2% |
27.8% |
75% |
47.2% |
47.2% |
|
Delta Sensitivity |
91.2% |
67.7% |
52.9% |
52.9% |
76.5% |
52.9% |
64.7% |
*Bekliz, Meriem, et al. “Sensitive of SARS-COV-2 Antigen-Detecting Rapid Tests for Omicron Variant.” MedRxiv,
https://www.medrxiv.org/content/10.1101/2021.12.18.21268018v2
Flowflex™ is authorized by FDA under an EUA and has the CE Mark for self-testing, is available in more than 100 countries around the world and is used by the National Health Service (NHS) in the United Kingdom as well as by governments and medical associations in the United States, Singapore, Australia, and many more. FlowFlex™ is suitable for use in individuals with and without COVID-19 symptoms, and for children as young as two years old.
FlowFlex™, which received its FDA EUA authorization* on Oct 4th, 2021, was instrumental to doubling the supply of rapid antigen test kits in the United States, having ramped up production to more than 200m test kits on a monthly basis.
“We are grateful to partner with ACON Bio, given the urgent need for rapid testing in Hong Kong. With our partnership, we aim to supply millions of testing kits and make it the most affordable FDA EUA authorized rapid test in Hong Kong,” said Danny Yeung, CEO and Co-Founder, Prenetics. “By providing near instant results without the need for specialist equipment or to visit testing facilities, Flowflex™ can provide reassurance before visiting family and friends, particularly those who are more vulnerable.”
To make it much more accessible to the general public, Flowflex™ will be priced significantly below the market average of HKD120 for other FDA EUA approved rapid testing solutions in Hong Kong.
The Flowflex™ Antigen Rapid Test Kit will be made available in the following packs:
1 Pack – HKD 75
5 Pack – HKD 350 (70 / test)
25 Pack – HKD 1500 (60 / test)
About Prenetics
Founded in 2014, Prenetics is a major global diagnostics and genetic testing company with the mission to bring health closer to millions of people globally and decentralize healthcare by making the three pillars — Prevention, Diagnostics and Personalized Care — comprehensive and accessible to anyone, at anytime and anywhere. Prenetics is led by visionary entrepreneur, Danny Yeung, with a team of over 800 employees and operations across 9 locations, including United Kingdom, Hong Kong, India, South Africa, and Southeast Asia. Prenetics develops consumer genetic testing and early colorectal cancer screening; provides COVID-19 testing, rapid point of care and at-home diagnostic testing and medical genetic testing. To date, Prenetics has processed more than eight million PCR laboratory tests for COVID-19 globally. To learn more about Prenetics, visit http://www.prenetics.com/.
About Flowflex™
Flowflex™ SARS-CoV-2 Antigen Rapid Test Kit identifies COVID-19 proteins and is certified to detect the Omicron variant and other variants. The easy-to-use at-home test provides results in just 15 minutes with 98.8% accuracy, 97.1% sensitivity and 99.5% specificity. Using a simple nasal swab within 2cm of the nose makes it extremely easy to administer an accurate test.
The test is authorized by FDA under an EUA, has the CE Mark for self-testing and has Singapore Health Service Authority HSA Pre-Market Approval (PMA) through HSA Pandemic Special Authorization Route (PSAR). It is available in over 100 markets and is already used extensively by governments and medical associations, including the Hong Kong government and the UK National Health Service. Flowflex™ was developed by ACON Bio, a US-headquartered company with over 27 years of experience in fields of high-quality diagnostics and medical devices.
Donate To The Indian Sun
Dear Reader,The Indian Sun is an independent organisation committed to community journalism. We have, through the years, been able to reach a wide audience especially with the growth of social media, where we also have a strong presence. With platforms such as YouTube videos, we have been able to engage in different forms of storytelling. However, the past few years, like many media organisations around the world, it has not been an easy path. We have a greater challenge. We believe community journalism is very important for a multicultural country like Australia. We’re not able to do everything, but we aim for some of the most interesting stories and journalism of quality. We call upon readers like you to support us and make any contribution. Do make a DONATION NOW so we can continue with the volume and quality journalism that we are able to practice.
Thank you for your support.
Best wishes,
Team The Indian Sun