Has significantly reduced side effects of the current diabetes drugs on the market
Maximize effectiveness by reducing drug and insulin resistance from long-term use
 |
SEOUL, South Korea, May 7, 2021 /PRNewswire/ — Nexturn Bio Inc., a subsidiary of Nexturn Bioscience Co., Ltd. (KOSDAQ:89140), said it has secured a 50% stake in RosVivo Therapeutics Inc., a U.S. new drug developer, as its first investment destination. RosVivo recently developed RSVI-301, a new drug pipeline that utilizes miRNA (MicroRNA), which is expected to be the next-generation drug, following mRNA (Messenger RNA), which has attracted attention as a Covid-19 vaccine. Unlike rare diseases or cancer, targeting chronic disease “diabetes,” seems to have positively affected Nexturn Bio’s investment.
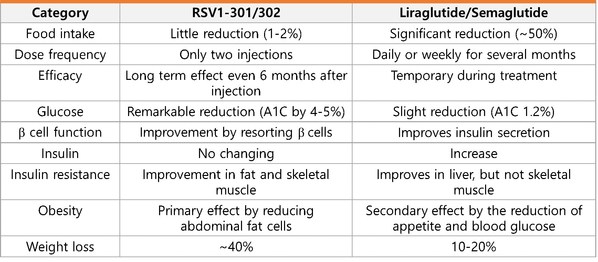
According to data released by the WHO in April this year, the number of diabetes patients worldwide is about 420 million, which has maintained a high prevalence rate until recently. The figure of the patients proves that diabetes drugs currently developed up to the fourth generation are not as effective as expected. In fact, Liraglutide, which are known to be the most effective component of diabetes drugs ever approved by the FDA, have also reported vomiting, diarrhea, constipation and indigestion as side effects from the clinical stages. The side effect reduction of RSVI-301 is more noticeable in the table below, compiled according to the research paper provided by RosVivo. (figure 1).
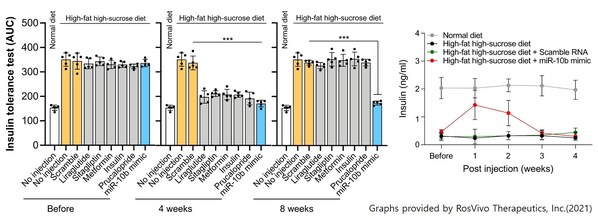
Furthermore, it has been observed that RSVI-301 reduces insulin resistance by normal levels during repeated drug infusions (figure 2). With the result of being completely opposite to the existing diabetes drugs, the possibility of overcoming insulin resistance raises RSVI-301 expectations in the related industry. The low side effects and the long-term lasting effects of RSVI-301 are expected to play a major competitive role in the future diabetes market in regard to patient convenience. An official from Nexturn Bio Inc. added, “We will continue to provide generous support for RosVivo’s development of new drugs as well as to cure diabetes together.”
Donate To The Indian Sun
Dear Reader,The Indian Sun is an independent organisation committed to community journalism. We have, through the years, been able to reach a wide audience especially with the growth of social media, where we also have a strong presence. With platforms such as YouTube videos, we have been able to engage in different forms of storytelling. However, the past few years, like many media organisations around the world, it has not been an easy path. We have a greater challenge. We believe community journalism is very important for a multicultural country like Australia. We’re not able to do everything, but we aim for some of the most interesting stories and journalism of quality. We call upon readers like you to support us and make any contribution. Do make a DONATION NOW so we can continue with the volume and quality journalism that we are able to practice.
Thank you for your support.
Best wishes,
Team The Indian Sun